how to draw fischer projections from line structures
25.2: Depicting Carbohydrate Stereochemistry - Fischer Projections
- Page ID
- 36454
Objectives
After completing this department, you should be able to
- draw the Fischer projection of a monosaccharide, given its wedge and nuance construction or a molecular model.
- draw the wedge and nuance structure of a monosaccharide, given its Fischer projection or a molecular model.
- construct a molecular model of a monosaccharide, given its Fischer project or wedge and dash structure.
Make certain that y'all tin can define, and use in context, the key term below.
- Fischer projection
When studying this section, use your molecular model set up to assist you in visualizing the structures of the compounds that are discussed. Information technology is important that yous be able to make up one's mind whether ii plainly different Fischer projections correspond two different structures or one single structure. Often the simplest manner to bank check is to construct a molecular model corresponding to each projection formula, and then compare the two models.
Cartoon Representations of 3D Structures
The problem of drawing three-dimensional configurations on a two-dimensional surface, such as a piece of newspaper, has been a long-standing concern of chemists. The wedge and dash notations we accept been using are effective, but tin exist troublesome when applied to compounds having many chiral centers. As part of his Nobel Prize-winning research on carbohydrates, the great German chemist Emil Fischer, devised a simple notation that is still widely used. Fischer Projections allow us to correspond 3D molecular structures in a 2D environment without changing their properties and/or structural integrity.
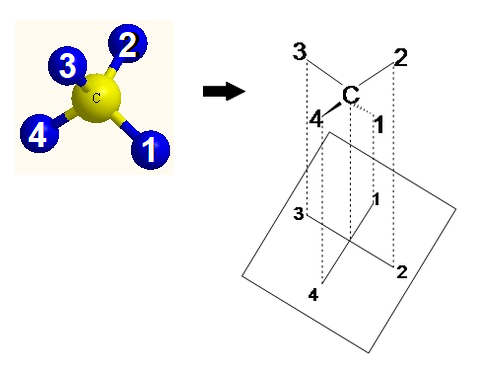
Fisher projections bear witness sugars in their open chain form. In a Fischer project, the carbon atoms of a sugar molecule are connected vertically past solid lines, while carbon-oxygen and carbon-hydrogen bonds are shown horizontally. Stereochemical data is conveyed by a unproblematic rule: vertical bonds point into the plane of the page, while horizontal bonds point out of the folio.
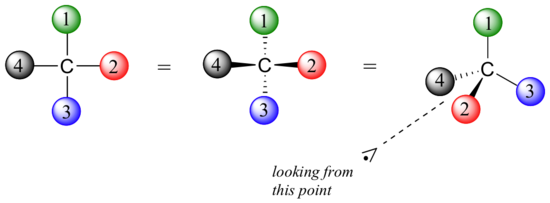
Sugars can exist drawn in the direct chain form as either Fisher projections or perspective structural formulas. When drawing Fischer projections, the aldehyde group is written at the top, and the H and OH groups that are fastened to each chiral carbon are written to the right or left. The arrangement of the atoms distinguishes 1 stereoisomer from the other.
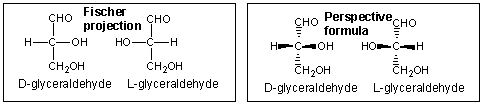
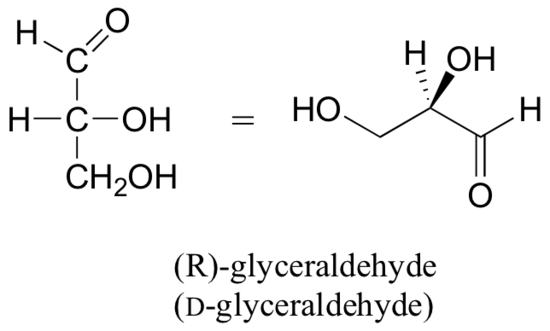
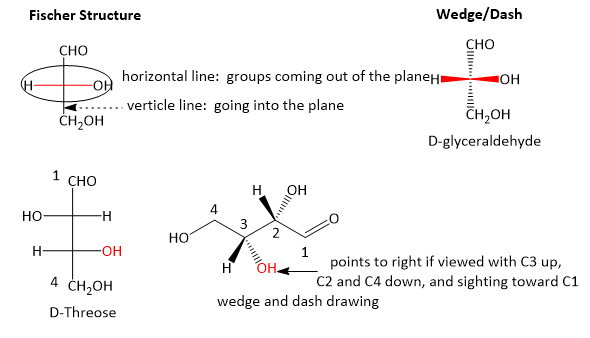
Beneath are two dissimilar representations of (R)-glyceraldehyde, the smallest saccharide molecule (also called D -glyceraldehyde in the stereochemical nomenclature used for sugars):
In the Fisher projection, the vertical bonds point down into the plane of the newspaper. That'south easy to visualize for 3C molecules. but more complicated for bigger molecules. For those draw a wedge and dash line drawing of the molecule. When determining the orientation of the hydroxides on each C, orient the wedge and dash drawing in your heed so that the C atoms next to the one of interest are pointing downwardly. Sighting towards the carbonyl C, if the OH is pointing to the right in the Fisher project, it should exist pointing to the right in the wedge and dash cartoon, every bit shown below for D-erthyrose and D-glucose.
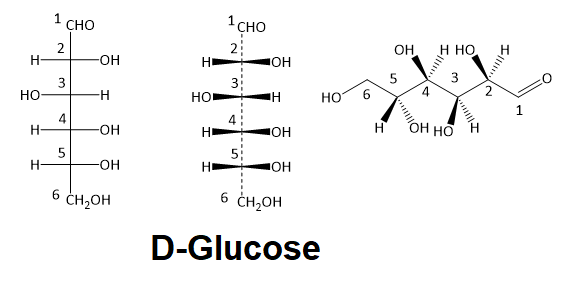
Below are iii representations of the open up chain form of D -glucose: in the conventional Fischer project, a wedge/dash version of a Fischer project, and finally in the 'zigzag' style that is preferred by many organic chemists.
Fischer projections are useful when looking at many different diastereomeric sugar structures, considering the eye can quickly selection out stereochemical differences according to whether a hydroxyl group is on the left or correct side of the structure.
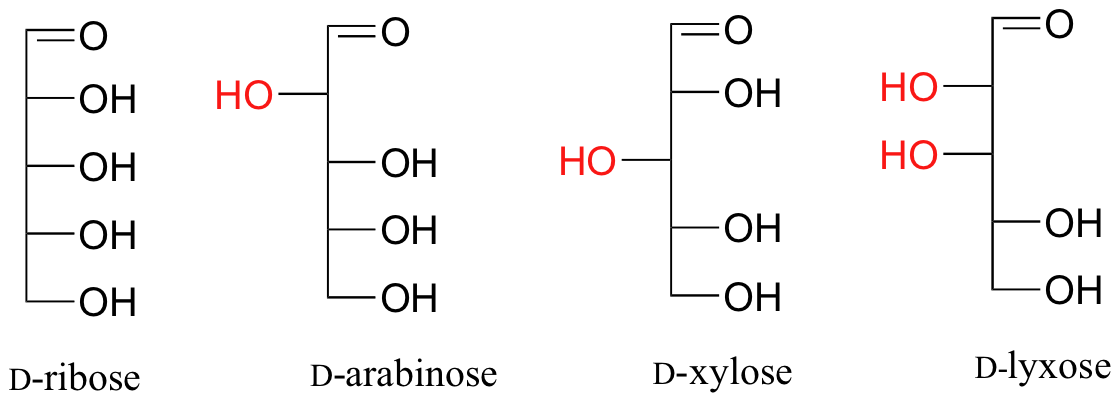
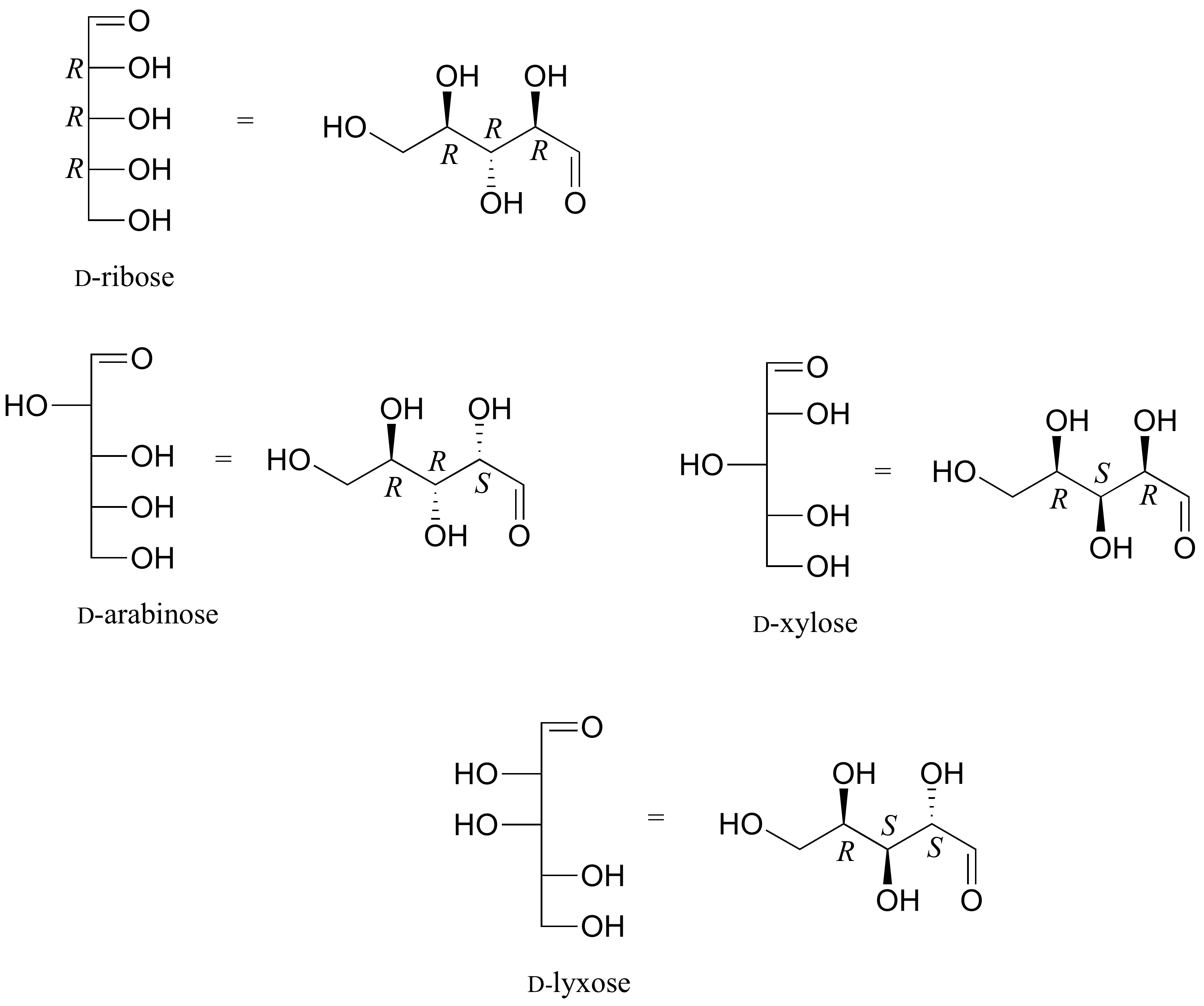
The usefulness of this annotation to Fischer, in his saccharide studies, is evident in the post-obit diagram. In that location are eight stereoisomers of 2,3,4,5-tetrahydroxypentanal, a group of compounds referred to every bit the aldopentoses (aldo- since the oxidized carbon is an aldehyde and -pentose since the molecules contain 5 carbons). Since in that location are three chiral centers in this constitution, we should expect a maximum of 23 stereoisomers. These 8 stereoisomers consist of four sets of enantiomers. If the configuration at C-iv is kept constant (R in the examples shown here), the four stereoisomers that result volition be diastereomers.
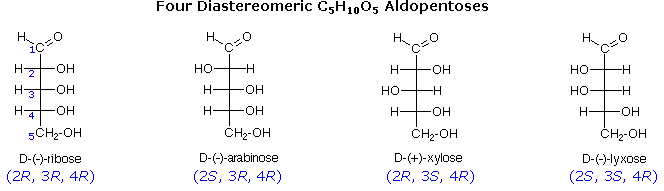
The aldopentose structures drawn above are all diastereomers. A more selective term, epimer, is used to designate diastereomers that differ in configuration at only 1 chiral center. Thus, ribose and arabinose are epimers at C-2, and arabinose and lyxose are epimers at C-three. However, arabinose and xylose are not epimers, since their configurations differ at both C-2 and C-3.
The Fisher structures of the most common monosaccharides (other than glyceraldehyde and dihydroxyacetone), which you volition see almost oftentimes are shown below.
Determining R and S in Fischer Projections
Determining whether a chiral carbon is R or South may seem hard when using Fischer projections, simply it is really quite unproblematic. If the lowest priority group (oft a hydrogen) is on a vertical bond, the configuration is given straight from the relative positions of the three higher-ranked substituents. If the everyman priority group is on a horizontal bond, the positions of the remaining groups give the wrong answer (you are in looking at the configuration from the incorrect side), so you simply reverse it.
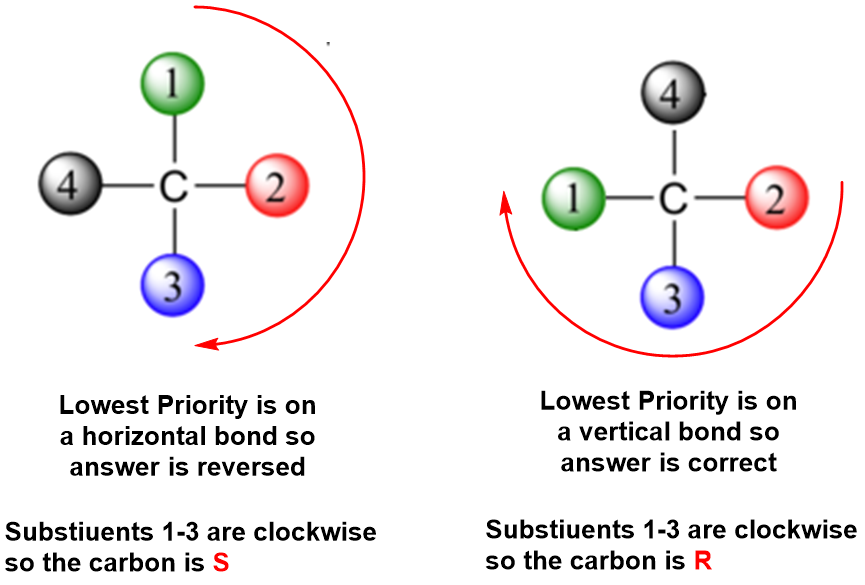
Worked Example \(\PageIndex{1}\)
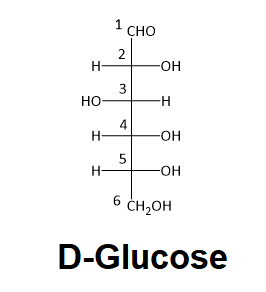
Decide if carbon #two in D-glucose is R or S.
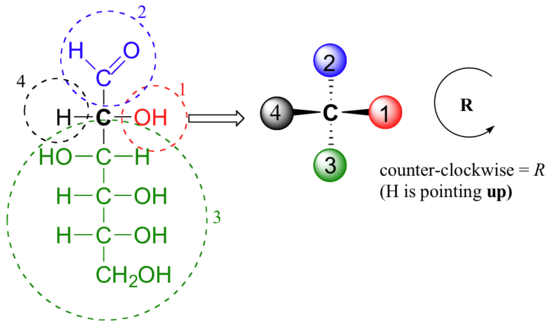
- Answer
-
When deciding whether a stereocenter in a Fischer projection is R or South, realize that the hydrogen, in a horizontal bail. Therefore, the orientation of the 3 remaining substituents is reversed to create the correct answer or a counterclockwise circle means R, and a clockwise circle means Due south. For carbon #ii in D-Glucose substituent ane, 2, and 3 form a counterclockwise circle so the carbon is R.
How to make Fischer Projections
To make a Fischer Projection, it is easier to show through examples than through words. Lets starting time with the first example, turning a 3D structure of ethane into a 2D Fischer Projection.
Worked Example \(\PageIndex{2}\)
Offset past mentally converting a 3D structure into a Dashed-Wedged Line Structure. Remember, the atoms that are pointed toward the viewer would be designated with a wedged lines and the ones pointed away from the viewer are designated with dashed lines.
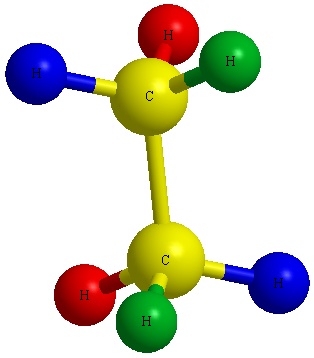
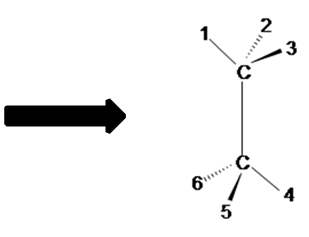
Discover the carmine balls (atoms) in Figure A above are pointed away from the screen. These atoms will exist designated with dashed lines like those in Figure B past number 2 and 6. The green assurance (atoms) are pointed toward the screen. These atoms volition be designated with wedged lines similar those in Figure B by number iii and 5. The blue atoms are in the plane of the screen so they are designated with straight lines.
Now that we take our Dashed- Wedged Line Structure, nosotros can catechumen it to a Fischer Projection. However, before nosotros tin catechumen this Dashed-Wedged Line Construction into a Fischer Project, we must first convert it to a "flat" Dashed-Wedged Line Construction. Then from there we can draw our Fischer Project. Lets start with a more simpler example. Instead of using the ethane shown in Effigy A and B, we will start with a methane. The reason being is that it allows us to only focus on one central carbon, which make things a fiddling flake easier.
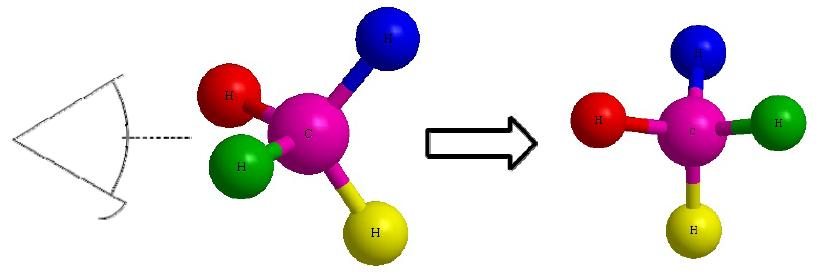
Lets offset with this 3D image and work our way to a dashed-wedged prototype. Start by imagining yourself looking directly at the central carbon from the left side as shown in Figure C. It should look something similar Figure D. Now take this Effigy D and flatten it out on the surface of the paper and you should go an epitome of a cantankerous.
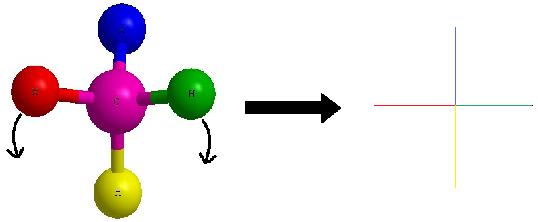
As a reminder, the horizontal line represents atoms that are coming out of the paper and the vertical line represents atoms that are going into the paper. The cantankerous image to the right of the arrow is a Fischer projection.
Exercise \(\PageIndex{ane}\)
one) Draw 'zigzag' structures (using the solid/dash wedge convention to show stereochemistry) for the iv sugars in the effigy below. Label all stereocenters R or Due south.
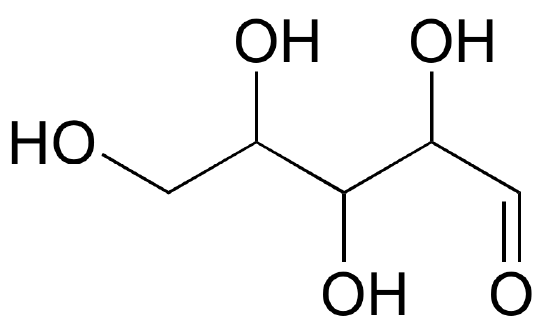
- Answer
- 1)
-
.
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/25%3A_Biomolecules-_Carbohydrates/25.03%3A_Fischer_Projections
Posted by: pappalardoyouten.blogspot.com


0 Response to "how to draw fischer projections from line structures"
Post a Comment